These days, all of our social issues seem to be connected to COVID-19 one way or another. Due to the COVID-19 outbreak that is lasting longer than anyone had expected, the world is suffering from social and economic hardships, not to mention the broken balance of life on a personal level. Everyone is wishing for COVID-19 vaccine and treatment drugs to come out as soon as possible, and the pharmaceutical and research institutes from all over the world are all geared towards the development of COVID-19 beaters. To develop vaccines and treatments, the safety and effectiveness of candidate drugs must be demonstrated through non-clinical and clinical trials. For diseases like COVID-19, the clinical trials must be conducted in a non-face-to-face method, so a higher reliability from relevant institutions is required.

CEO Lee Jeong-hee who runs the clinical trial support platform AllLiveC said, “To commercialize the treatment drugs, we have to run Phase 1-3 of the clinical trials in humans. And, for infectious diseases like COVID-19, the contact between the subjects and the investigators should be minimized, so we have many restrictions in recruitment and the clinical trial process.” AllLiveC is a platform operated by AllLive Healthcare, Korea’s no. 1 clinical trial support service provider. It is providing the clinical trial online/offline integrated brokerage service that connects applicants who want to participate and the clinical trial centers.
A smart platform
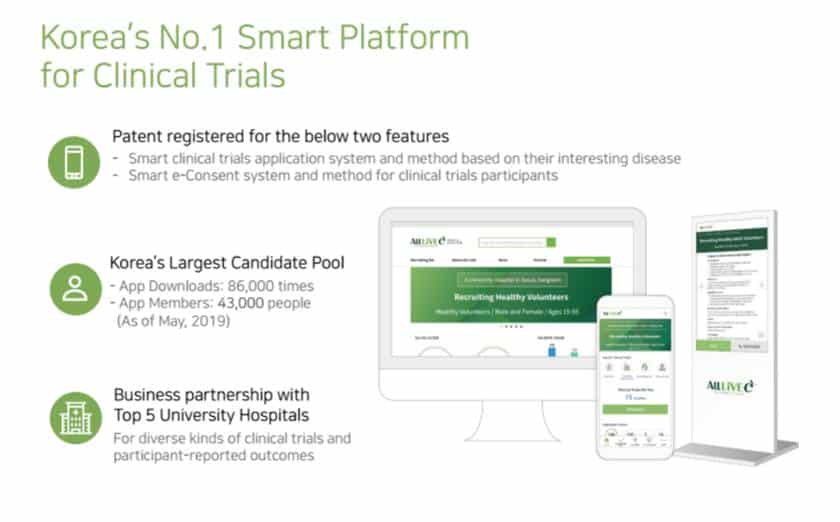
Clinical trial is the most important process in the development of new drugs. A huge amount of time and money is spent. AllLive Healthcare has the goal to innovate these clinical trials with IT technology. As the first step, it has made the platform that provides the service of recruiting and managing the subjects for clinical trials. By using the IT technology, the recruitment of clinical trial subjects that had been conducted manually is done by AllLiveC, the clinical online/offline clinical trial brokerage service. It is announcing the clinical trials by online media.
Under the motto of “becoming the chain that links the beginning and the end of new drug development and clinical trials,” AllLive Healthcare was established in 2015. Its main business targets are mainly the pharmaceutical companies known as the sponsor, clinical trial centers known as the CRO, hospitals, and participants known as clinical trial applicants (patients, healthy subjects). To maximize the efficiency of recruitment, a professional call center is used. The company also provides other convenient services like total PR media planning and operation, preparatory work for IRB reviews, recruitment of participants, and analysis of the advertising media in its packaged solution AllLiveC One-stop Service.
Tested and ready
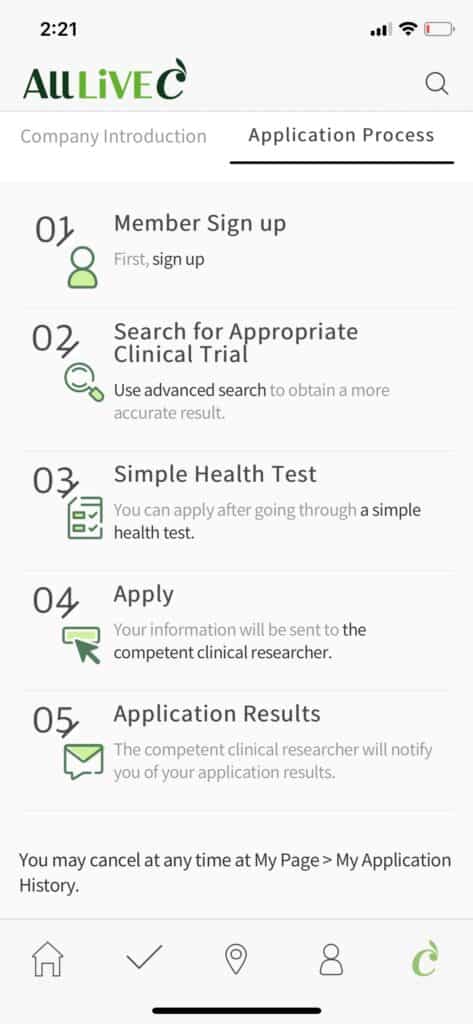
CEO Lee has witnessed many failures of startups as he was in charge of incubation and acceleration that discover and develop startup companies. He emphasizes that for the companies to enter the market with stability and continue to grow, market trends should be interpreted accurately. He is continuously investing in IT-based R&D to expand the efficiency and convenience of conducting clinical trials in line with market trends. Along with this, he is developing his own competitiveness of AllLive Healthcare by identifying the market trend and using an innovative expert group to lead the market and the experience of working with 100 pharmaceutical companies and 150 clinical trial centers.
In clinical trials for infectious diseases such as COVID-19, AllLiveC, a smart clinical trial support platform that uses the technology of a non-face-to-face automated consent system shows its true value. He explains, “We have continued the research of integrating non-face-to-face technology to clinical trials since 2018. We have completed the technology development for non-face-to-face automated consent system. It can be used in COVID-19 clinical trials right away.”
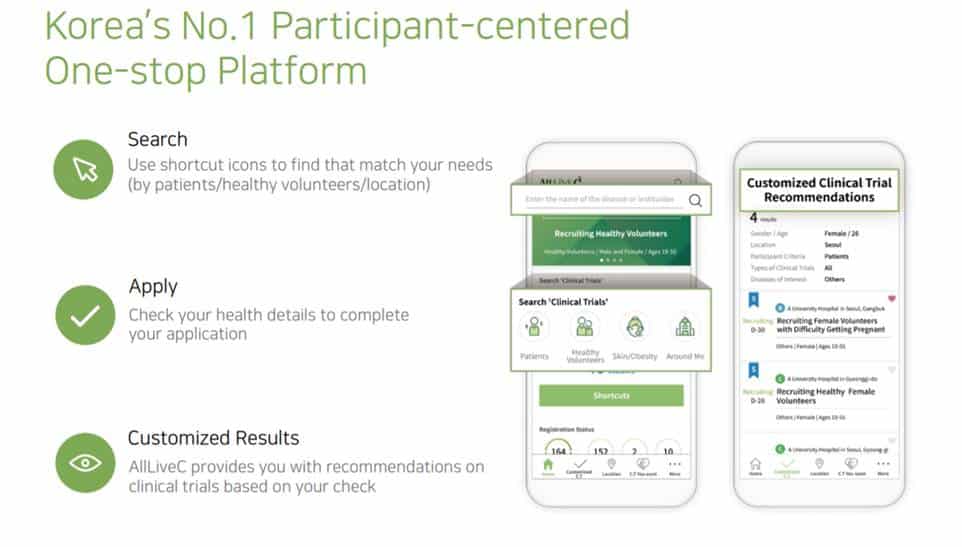
AllLive Healthcare has been developed in a multilingual version last year, and a pilot project is being conducted in Singapore. Lee says, “There is a limit to expanding business in the global market with applications alone. That’s why we are planning to connect fully automated or remote clinical systems to the application before entering the overseas market.” The company’s goal is to take one step up from the business that just recruits and manages clinical trial applicants, and evolve as the company that provides a remote clinical trial system by combining the IT and AI technologies. It has the dream to become a “Digital CRO” that continuously improves the efficiency of the clinical trial industry by acting as the pharmaceutical companies and hospitals, and supporting the clinical trial faster and more accurately.
About Pangyo

For AllLive Healthcare, being located in Pangyo not only provides the geographical advantage of being the center of the metropolitan area, but also the chance to receive infinite opportunities of cooperation by having IT companies of various fields and bio companies close by. Lee thinks, “Pangyo is a place of integration between the IT ecosystem and the bio ecosystem, which makes this place the best place to start the business. Also, relevant organizations lie Korea Bio is also located in Pangyo, so we are utilizing it for networking. Not only that, we also have the advantage of recruiting great human resources like the developers and designers.”
Interested about Pangyo? find more articles here.
